In this Newsletter
The IRB by the Numbers
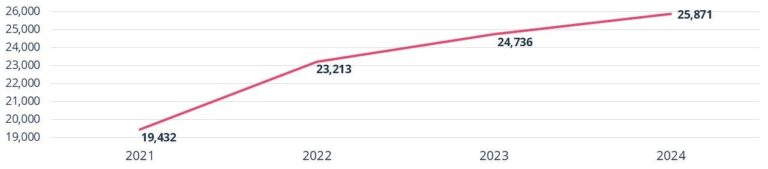
Total Number of IRB Submissions
- 2021: 19,432
- 2022: 23,213
- 2023: 24,736
- 2024: 25,871
Median Turnaround Times for Study Reviews (Jan – July 2025)
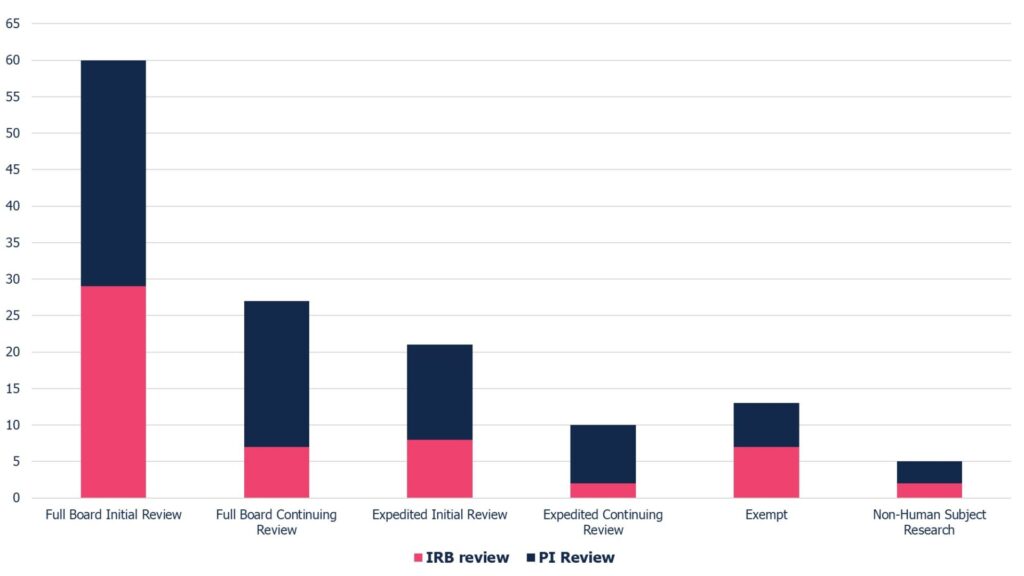
| Full Board Initial Review: 60 days IRB Court: 29 days PI Court: 31 days | Expedited Initial Review: 21 days IRB Court: 8 days PI Court: 13 days | Exempt: 13 days IRB Court: 7 days PI Court: 6 days |
| Full Board Continuing Review: 27 days IRB Court: 7 days PI Court: 20 days | Expedited Continuing Review: 10 days IRB Court: 2 days PI Court: 8 days | Non-Human Subject Research: 5 days IRB Court: 2 days PI Court: 3 days |
Median Turnaround Times for Initial Rely On Study Reviews (Jan – July 2025)
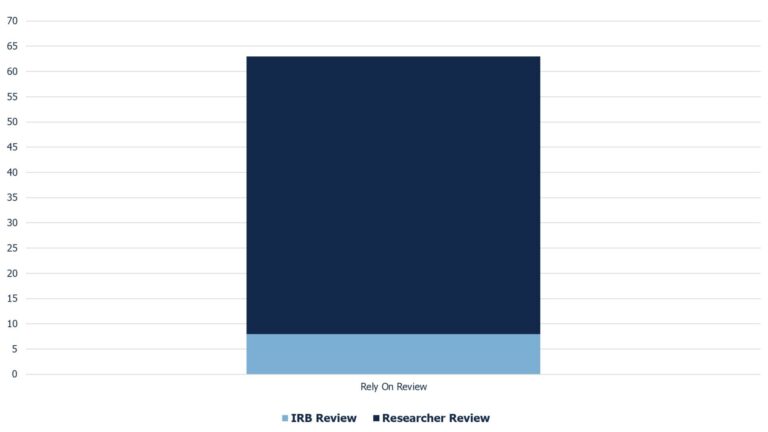
| Rely On Review: 63 days IRB Court: 8 days PI Court: 55 days |
News
New Opportunity for PI Participation in Full Board IRB Review
The Office of Human Research Ethics (OHRE) now invites Principal Investigators (or their designee) to attend the IRB meeting when their initial study submission is reviewed by the full board.
OHRE will email the study team with an invitation, assign a specific time during the meeting, and provide a Zoom link. Researchers will be placed in the virtual waiting room until the board completes its initial discussion, then join briefly to address specific questions. Early feedback suggests this approach is improving communication and helping the board assess whether the Criteria for Approval are met, thereby reducing the number of studies that are deferred.
This new process helps clarify aspects of the research that might otherwise result in deferral. Questions may be directed to Administrative Support Specialist, Beverly Malone.
Updated HIPAA Authorization Form
As of May 9th, in consultation with the Privacy Office, the HIPAA authorization form has been updated to better define the entities that will receive participants’ PHI. The form also contains a new section to include the purpose of the study and describe how participants’ health information will be used to accomplish the aims of the study. This is necessary in order to address ongoing concerns with contracts and agreements with Sponsors and other third parties.
Please access the updated authorization template from the “Consents” section of the OHRE website for all new applications. Previously reviewed and approved HIPAA forms do not need to be updated.
New Ticketing System to Request Help from OHRE
UNC OHRE will move all internal inquiries to TeamDynamix (TDX) and will sunset the use of [email protected]. Going forward, please submit your IRB questions using our new ticketing system. This move will enable us to better track the questions that you have so that we can update our guidance on the website to align with the needs of our investigators.
From the TDX site, you will be able to submit inquiries for Education, Reliance, Promptly Reportable Information, and IRB Operations. For IRB Operations questions, you can also access the link using the Help tab in IRBIS.
New Quality Assurance and Improvement Program
The OHRE Quality Assurance and Improvement Program has begun conducting routine informed consent observations across campus. These reviews are designed to be collaborative and educational, supporting our shared mission to protect the welfare of research participants at UNC and strengthen partnerships with our research community.
If your study is selected, a member of the IRB Quality Improvement team will meet with you one-on-one at your site to:
- Observe the informed consent process from start to finish
- Answer questions and discuss opportunities for process improvement
- Provide tailored recommendations and support
Coming Soon: Tip sheets, resources, and answers to frequently asked questions will be available on our website to help guide and enhance your consent practices.
New OHRE Website Layout
OHRE has updated and reorganized our website. Some pages have been moved, combined, or eliminated to make the browsing experience faster and easier. If you have bookmarked OHRE webpages, please check that the links are still active.
If you need help finding something, email Education Content Manager, Eric Schumacher.
Laura Munn Celebrates 30 years at UNC!
Business Services Coordinator Laura Munn is celebrating 30 years at UNC and 23 years at OHRE!
Laura has been a reliable and friendly presence at UNC since her first day in 1995. Whether juggling tough schedules or mentoring new employees, Laura has always gone the extra mile. Her unwavering commitment, expertise, and spirit of collaboration have helped make OHRE what it is today.
Please join us in congratulating Laura on her incredible journey and service!

Guidance
New Published Guidance on Certificates of Confidentiality
Visit OHRE’s website for new published guidance on navigating Certificates of Confidentiality. A Certificate of Confidentiality protects the privacy of research participants enrolled in biomedical, behavioral, clinical or other types of health-related research that collect or use identifiable, sensitive information. The Certificate prohibits disclosure in response to legal demands, such as a subpoena.
The new guidance page has sections for Usage, CoC Application Procedures for Non-Federally Funded Research, Limitations, Amending Certificates of Confidentiality, Expiration of Certificates of Confidentiality, and References and Resources.
Best Practices for Naming Files in IRBIS
IRBIS uses SharePoint to manage documents. It’s important for you to update the names of edited files with a version number or version date because SharePoint can’t tell the difference between multiple files if they have the same name.
See OHRE’s best practices for naming your files in a clear and consistent way.
Best Practices for Stamped Consent Forms
OHRE implemented date stamping of consent forms in May 2024. Since that time, we have noted some instances where the stamp fails to be placed or is obscured by other text. Please follow the steps linked below to ensure that all consent forms are stamped appropriately. Note that only stamped consent forms should be used when obtaining consent.
See OHRE’s best practices for stamped consent forms.
Meet the Chairs
David Weber, Chair of Committee A
Dr. Weber is chair of IRB A, and chairs three Pharmacy Committees. His research interests include the epidemiology of healthcare-associated infections, disinfection and sterilization, new and emerging infectious diseases (highly communicable pathogens including SARS-CoV-2, HPAI, newly emerging pathogens including Candida auris), response to biothreats, nontuberculous mycobacteria, control of drug-resistant pathogens, immunization practices, zoonotic diseases, and epidemiology of tuberculosis.
In this article, Dr. Weber discusses the most interesting ethical issues he’s wrestled with at the IRB, explains his most common reasons for deferring a study, and gives advice for researchers submitting to the IRB at UNC for the first time.

Education
An Overview of IRB Compliance and SWAG
What is compliance? OHRE’s new video outlines the compliance review process, the composition of the Safety and Welfare Analysis Group (SWAG), and their role in the process, and the impact of SWAG on promoting efficiency while ensuring a quality review of an event.
Visit OHRE’s Training and Education Resources page to see all our online training offerings.
Small, Medium, or Large? Customizing and Emphasizing the Need for Engaging IRB and IACUC Member Training to Build and Maintain Stability
OHRE staffers, Kim Brownley (Associate Director, Policy and Initiatives), Charlotte Coley (Education/Training Manager), and Eric Schumacher (Education Content Manager) recently led an education session for PRIM&R alongside Jess Marshall from Mississippi State. The presentation describes how OHRE and Mississippi State provide continuing training for IRB and IACUC members and staff, with a focus on organizing retreats and developing digital materials.
Feedback was very positive. One attendee said, “I appreciate the specific examples they provided on how they make the high-quality training & educational materials for the IRB members & research community. The scope of content and quality of materials that the UNC team have created is impressive.”
The session is available to view from PRIM&R for a fee.
Contact Us
Have Questions?
Fill out a ticket or call us at 919-966-3113 for general questions. Visit our contact page for specific inquiries
Register for Upcoming Office Hours with an IRB Analyst
Give us Feedback
We’d love to hear from you! Tell us what you think with an anonymous survey. It will take you less than five minutes to complete.