The IRB Meetings and Deadlines Calendar has been published for 2021 on the OHRE website. With the restructuring of the boards to remove the designations of Biomedical, Non-biomedical, and Safety last year, all submissions are assigned to the board agendas based on the submission date. Please note that receipt by the submission deadline does not guarantee review at the corresponding meeting. Once a full board agenda is full, it is closed; subsequent submissions are assigned to the next available agenda.
You may notice that some deadlines are shared between two agendas. This occurs when two meetings are proximate and allows more flexibility to assign the submissions to the board with the appropriate expertise, when needed, and to accommodate expiration dates.
You can check the date that your study will be reviewed by the IRB Committee. On your study’s “IRB Study Management” page, the “Full Board Agenda” column will display “n/a” during the Administrative Pre-Review. Once your submission is assigned to an agenda, the agenda date will be displayed.
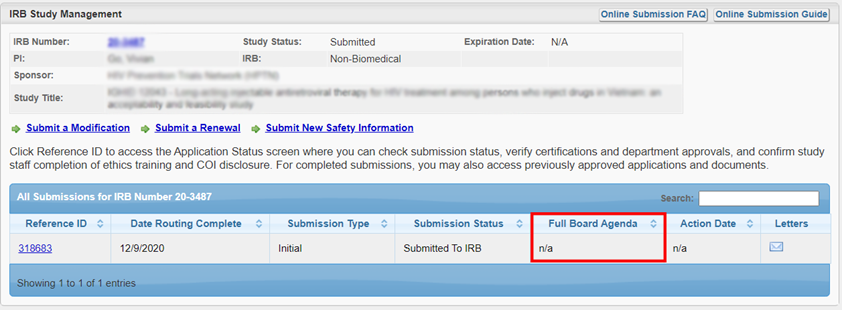
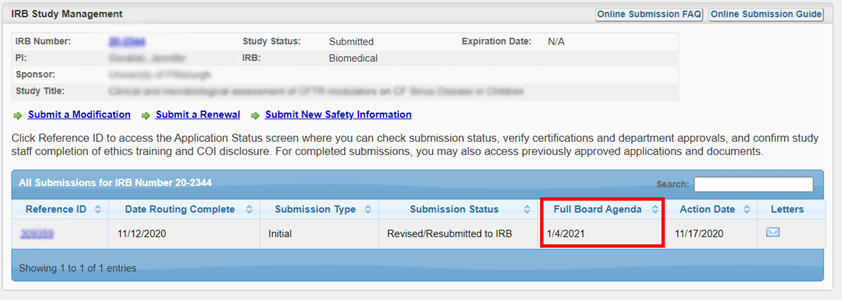
For questions about your submission, you can reach out directly to the analyst assigned. On the top of the submission page, the analyst is listed. You can access contact information from the hyperlink.

