How to disinfect to kill a virus
Download a printable PDF of this infographic.
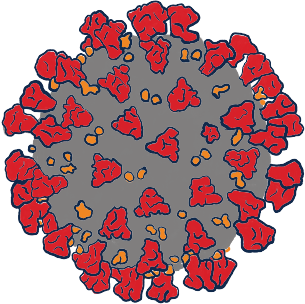
A virus is a microbe that is capable of infecting humans by taking over their cells and changing how the cells behave.


Cleaning is removing visible dirt, while disinfecting uses specific products to kill invisible viruses on surfaces. Many of these products can be found in the home.
Disinfect surfaces that you come into contact with, such as:




If bleach solution irritates the skin, itʼs likely too strong.
NEVER mix any of these products together, only mix with water.
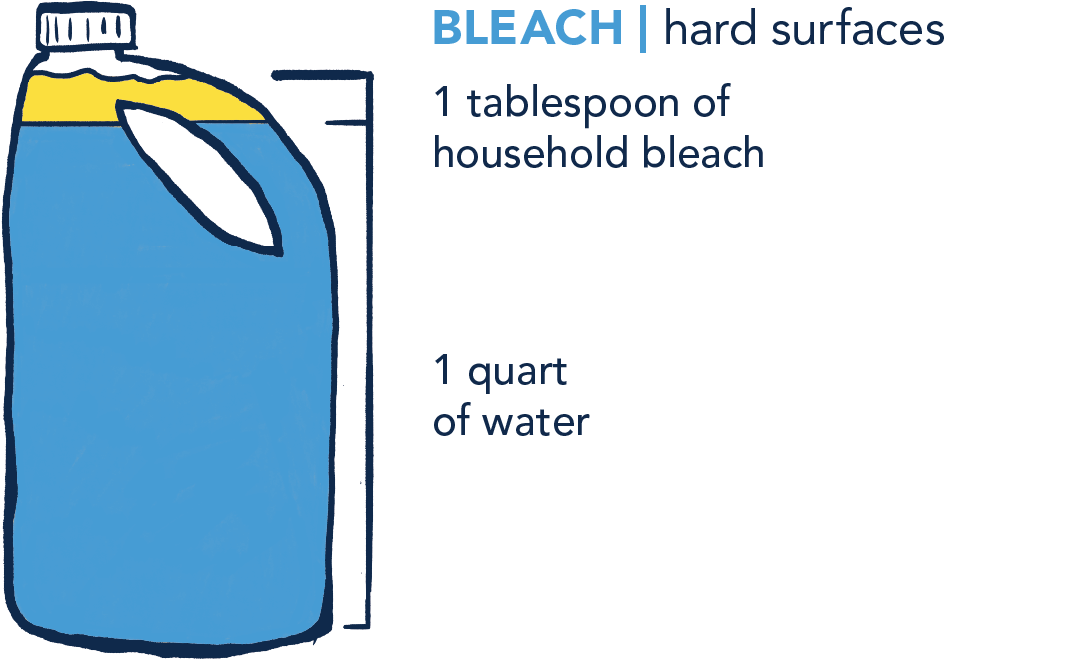
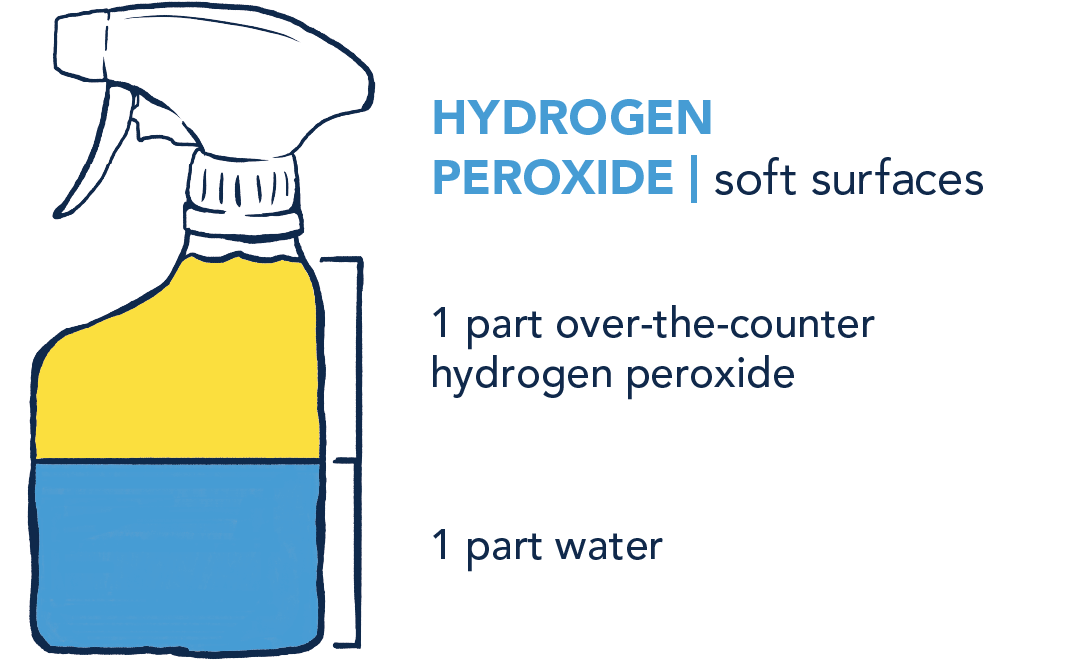


- Apply any of these three mixes to surfaces, let stand 5–10 minutes, then wipe down.
- For hydrogen peroxide, make small fresh batches daily.
- Label your bottles and reuse the bottles for the same disinfectant over time.







