Some of the scariest complications in pregnancy involve preeclampsia, the primary cause of maternal mortality worldwide.
The disorder leads to high blood pressure and organ damage, and the only treatment is to deliver the baby regardless of how far along the pregnancy is. Though scientists do not fully understand why or how preeclampsia occurs, they know that part of the problem stems from the fertilized egg’s inability to implant properly in the wall of the mother’s uterus.
Kathleen Caron and her team of researchers identified a gene involved in implantation. Their findings will advance research into reproductive disorders, such as preeclampsia, miscarriage, and infertility.
These types of pregnancy complications are common, with preeclampsia affecting up to 8 percent of pregnancies. As many as 50 percent of pregnancies are thought to end in miscarriage, and 10 percent of women in their reproductive years experience infertility.
Published clinical studies have implicated andrenomedullin, a small protein, in several types of pregnancy complications, Caron says. But so far the studies can only imply an association, not a causal relationship, between the protein and these complications.
Caron’s study is different. “We provide proof of principle,” she says.
Adrenomedullin widens blood vessels and helps form new ones, likely assisting the implantation process by allowing more blood flow to the site, Caron says. A deep implantation is crucial to the nourishment of a developing embryo.
During a typical pregnancy, adrenomedullin levels increase about fivefold in the mother. “Both the mother and the fetus are making remarkable attempts to increase the level of adrenomedullin secretion right at the site of implantation,” Caron says.
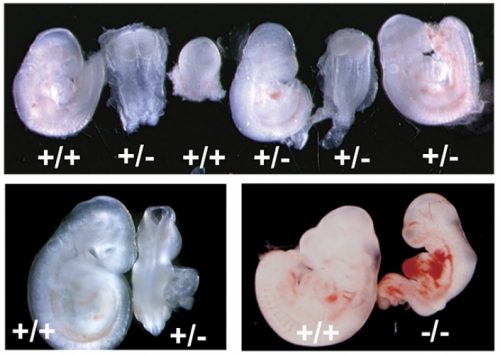
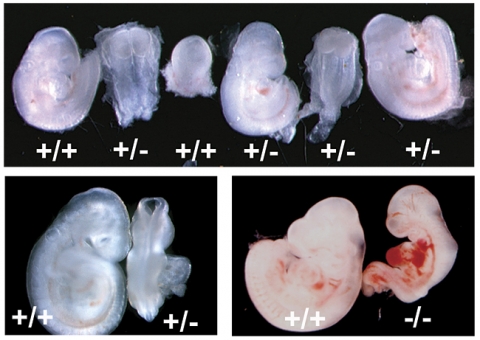
When researchers genetically manipulated mice to secrete slightly reduced levels of adrenomedullin, they uncovered a lot of problems with implantation, Caron says.
Instead of implying a relationship between adrenomedullin and implantation problems like previous studies did, this study showed that mutating the gene that controls adrenomedullin secretion actually caused those problems.
The researchers found that the mice with reduced adrenomedullin levels had smaller litter sizes. Embryos were not well burrowed into the uterus, and they were spaced too closely together, restricting their growth.
These symptoms — decreased fertility, shallow implantation, and reduced fetal growth — are all associated with preeclampsia and other fertility problems in humans.
“Preeclampsia is probably caused by multiple genes because it affects so many women,” Caron says. “Adrenomedullin is one of those genes.”
Caron and her team plan to move their research from mice to humans. They’re also studying two types of proteins associated with adrenomedullin’s functions that may one day become targets for new drugs.
Julia Connors was formerly a student contributor to Endeavors.
Kathleen Caron is an assistant professor in the Department of Cell and Molecular Physiology in the School of Medicine. The study appeared in the October 2006 issue of The Journal of Clinical Investigation.