- One third of us have TB bacteria in our bodies. Worldwide, TB infects one new victim per second.
- The earliest unambiguous detection of the tuberculosis bacterium is in bison remains dated to 18,000 years ago.
- “In approaching the consumptive, one takes the disease because there is in the air something disease producing.” — Aristotle
- Hunchbacked figures that appear in Egyptian art tell us that tuberculosis has afflicted humans since at least 3,000 BC.
- TB was once the leading cause of death in the United States.
- In the 1970s and early 1980s, the United States neglected the TB control efforts that it had begun in the 1940s. Between 1985 and 1992, the number of TB cases increased.
- In 2005, 1.6 million people worldwide — more than 4,300 per day — died from TB.
- Because TB medications are meant to be taken all together, some patients have to swallow up to sixteen pills at a time.
- TB is the leading killer of people who have HIV. Active TB will develop in 30 percent of people living with AIDS.
- Tuberculosis is treatable and curable — even in people living with HIV.
- People who have latent TB infection do not feel sick, have no symptoms, and cannot spread TB to others. About 10 percent of people with latent infection will develop TB disease. Some people develop active TB disease soon after infection, before their immune systems can fight the bacteria. Others get sick later when their immune systems weaken for another reason.
- If a person’s immune system can’t stop TB bacteria from multiplying, the bacteria attack, destroying tissue and causing active TB disease. TB bacteria can create holes in a person’s lungs.
In May of 2007 a sick man took a flight from Atlanta to Paris. He got married in Greece, and was touring Rome when he got a call from the Centers for Disease Control and Prevention (CDC). Stay where you are, they told him. We’ll send a private jet to pick you up. The U.S. government had placed a no-fly order on his passport because, according to CDC test results, he had an extremely drug-resistant form of tuberculosis — XDR-TB — which is infectious and often untreatable.
The media went berserk. CNN gave hourly updates on the man. The CDC enacted its first federal quarantine in over forty years. But the man panicked. He slipped onto another flight, and by the time officials finally quarantined him he’d left a trail of exposure all the way from Italy to Canada to New York.
In the end, it turned out the man had MDR-TB, a less dire drug-resistant strain. He wasn’t as infectious as officials had feared, which was why he didn’t cause an outbreak over the course of his travels. But even so, says TB researcher Miriam Braunstein, “those kinds of events tell you that TB’s a real disease — a real threat.”
There are three things you should know right off the bat about tuberculosis and the bacteria that cause it:
1. A third of us on the planet — about two billion people — are infected with it.
2. Ninety percent of those who have it never show symptoms.
3. Most of the time, TB is curable.
Most people’s immune systems react to the threat of M. tuberculosis and keep the bacteria at bay. But a compromised immune system — which can come about through depression, HIV, or plain old age — can no longer fight off an infection.
At last count in 2006, there were 13,779 cases of TB reported in the United States. We carry only a fraction of one percent of the world’s infected people (85 percent are in Africa). Beginning with the onset of the AIDS epidemic, the number of new cases began to grow; there are now nine million every year.
Number three on the list above may be the biggest reason we’re still living with tuberculosis today. Scientists in the 1950s developed antibiotic drugs that could cure TB patients completely, and after that, doctors were no longer trained to recognize the symptoms, prisons did away with quarantine facilities, and funding for TB research dried up. We still use the same treatment for TB today that we did in 1955. We use the same method of diagnosis — the tuberculin skin test — that we did a hundred years ago. And we’ve never had a dependable vaccine. As a result, scientists say, treatments for tuberculosis have seen less progress than those for any disease in the past fifty years.
After decades of inconsistent drug supplies, drug shortages, and poor drug quality, certain strains of TB have mutated and now hold strong against what drugs we do have. The World Health Organization calls these strains “a grave public health threat.” As of February 2008, forty-six countries across the world had confirmed cases of XDR, including the United States.
Multidrug-resistant (MDR) TB is resistant to at least two first-line TB medications, and can take years to treat. Extremely drug-resistant (XDR) TB is MDR that is also resistant to three or more of the six second-line drugs; it’s difficult both to diagnose and to treat, says epidemiologist Annelies Van Rie. Most people who contract XDR have no chance of recovery.
The disease’s greatest weapon is how easily it moves from body to body. All it takes is one cough to launch microscopic clumps of bacteria into the air. If the person sitting in the next seat on the bus inhales the clump — or even a single bacterium — M. tuberculosis can set up shop. The bacteria are soon detected by scavenging immune cells called macrophages, which immediately attack. The macrophages ingest the bacteria in a process called phagocytosis, and then digest them much like a meal.
This attack usually spells the end of any invading germ, but M. tuberculosis has evolved methods to survive it. The bacteria do go through phagocytosis, but somehow they manage to halt the degradation process. In those first few weeks of infection, M. tuberculosis survives and multiplies happily inside the macrophages. Eventually the bacteria reproduce so prodigiously that they burst free from the macrophage and infect other macrophages.
No one knows exactly how M. tuberculosis manages to thwart the macrophages, but microbiologist Miriam Braunstein and members of her lab at UNC are trying to find out. Studying the basic science of M. tuberculosis and how it causes disease, Braunstein says, could help us obliterate it.
By the time the bacteria are running rampant among the macrophages, the immune system of a healthy person realizes what’s going on and taps another line of defense: T cells. These immune cells can detect infected macrophages and release cytokines, which work like an emergency alert system to trigger intense immune responses. T cells trap infected macrophages and quarantine them into bundles of immune cells called granulomas. Inside the granuloma, T cells activate the infected macrophages using cytokines so they can finish the job of killing the bacteria.
This business of invasion and escape is usually silent in people who are healthy — at worst, they feel tired or have minor cold symptoms — because the reactivated macrophages kill the infection. But sometimes one or two bacteria can evade even the T-cell response and survive undetected in the granuloma (again, how M. tuberculosis manages this is unknown). They can lie dormant there for decades — up to eighty years — until age or infection weakens the immune system. This is how TB in a sixty-year-old patient may have originated from an infection he got when he was twenty years old.
In the hubbub surrounding the smallpox vaccine in the early twentieth century, the buzz among scientists was that a TB vaccine was not far off. But it turned out that the BCG vaccine, first tested on humans in 1921, wasn’t the cure scientists had hoped for.
BCG is made from a relative of TB called M. bovis, which lost its virulence after scientists grew it repeatedly on potato media. While the BCG strain doesn’t cause disease in humans, the idea was that it would help human immune systems prepare for future TB infection by introducing something similar to the real thing.
But the main problem with BCG is that it doesn’t work for everyone; in some studies it has protected only sixty to eighty percent of people, and in other studies, it provided no protection at all. And since antibiotics to treat TB were discovered just over twenty years after the BCG vaccine was developed, no one bothered to create a more effective vaccine. Today some countries require the BCG vaccination despite its shortcomings, but many scientists and doctors — including those in the United States — don’t even consider it to be a vaccine.
Braunstein studies how M. tuberculosis delivers proteins to cause the disease. “Most virulence factors of pathogens are secreted proteins,” she says, and so she began by looking at proteins secreted by the bacteria while they’re in the macrophage. Understanding this part of the bacteria’s process could help us develop a more effective vaccine, she says.
In 2003 Braunstein made a huge breakthrough: she found the specialized protein secretion system in M. tuberculosis called SecA2. Protein secretion systems are common in bacteria and work like mechanical ports that oversee what is allowed to leave the cell.
But scientists had never seen this system before, and no one had any idea what role it played in the disease. When Braunstein made a mutant strain of M. tuberculosis that lacked the SecA2 system, she found that it caused less disease in mice. “It also protected animals from TB infection better than BCG,” she says. The SecA2 mutant has now been licensed by a not-for-profit TB vaccine development company called Aeras that aims to use it in their efforts to make a more effective vaccine. If they’re successful, they’ll distribute it for the lowest possible cost.
“I like the fact that basic-science research can have very big implications in serious diseases,” Braunstein says. “I thought, ‘How did this protein get secreted?’ and now it may be a key to a new vaccine. When you’re not looking to cure the disease, you may come across something that will cure the disease.”
Braunstein and members of her lab continue to study SecA2, and the project is gaining momentum now that she’s working with professor of molecular pharmaceutics Tony Hickey. Hickey’s working to find the best way to get TB drugs (and eventually a vaccine) into the bodies of TB patients.
Pills haven’t been completely effective in the past, Hickey says. And with needles, there are the problems of storage, disposal, and making sure they’re not used more than once. So he started off by asking himself: if pulmonary TB attacks the lungs, and transmission occurs through inhalation, wouldn’t it be more effective to deliver the medicine straight to the lungs? He’s been working to unravel this question for almost twenty years. And according to his studies, the answer is yes.
But it’s not just a simple matter of inhaling BCG. Scientists tried that back in the 1960s, and the results were inconclusive. The problem, Hickey says, was one of delivery. So he used a combination of chemistry, biology, and physics to try to make it work. “You have the chemistry, which is largely physical chemistry and particle-particle interactions,” he says. “And then the physics of taking the particle and putting it into an air stream and delivering that into the lungs, which is all based on aerodynamic behavior of small particles. And then the biology — whether or not you actually target the microorganism you’re trying to kill to treat the disease.”
So now Hickey is adapting technology from certain types of aerosol delivery devices, such as asthma inhalers. “But there are some subtleties to protein stabilization. Most of the asthma compounds are very small molecules that are reasonably stable, so you don’t have to do much but make them into a particulate form.” With TB medications and vaccines, it’s tougher. Hickey wants to turn BCG into a powder (rather than a mist) that patients can inhale without having to use water. This will be especially useful in countries where clean water is scarce.
Right now Hickey and Braunstein are testing which TB antigens generate an immune response in guinea pigs. “Miriam and I are working on a protein-antigen aerosol for TB. There are all sorts of proteins and nucleic acids that the bug expresses that the body will generate an immune response to,” he says. “But there needs to be a lot more basic science to develop an effective new treatment.”
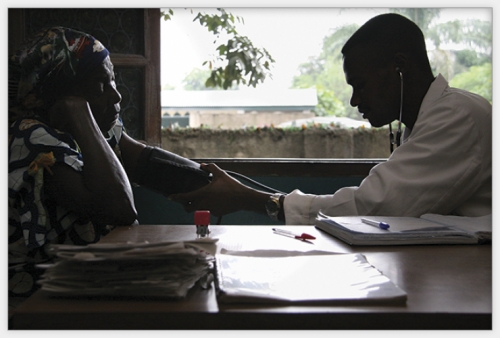
Although funding for TB research is flowing to scientists once again, they’re struggling to manage some new complications of the disease — namely HIV. TB is now the leading cause of death in people with HIV, and the two diseases have created what Annelies Van Rie calls a deadly symbiosis. Van Rie treats TB patients in South Africa, which accounts for 29 percent of the continent’s coinfections. “HIV drives the TB epidemic in sub-Saharan Africa,” Van Rie says. “So if you want to do something nowadays about TB there, you can’t neglect HIV.”
One of the biggest roadblocks to containing the epidemic is that many African health-care workers are only trained to treat one of the diseases. Nurses in the hardest-hit African countries have come under enormous pressure in recent years. Without access to continuous medical education (which doesn’t exist in their countries), their training is often haphazard, and many are never trained again during their careers. They also aren’t paid well and their working conditions are often terrible, Van Rie says; many die from HIV or move away to escape the risks. “The workload in sub-Saharan Africa has just increased,” she says, “but the nurses don’t increase.”
One of Van Rie’s latest research projects involved working with health-care workers at eleven clinics in the Democratic Republic of Congo. With all the new demands on the clinics, doctors and nurses were overwhelmed, and insisted they’d need additional staff and money to make ends meet. But after assessing how the clinic workers were using their time, Van Rie found that the TB nurses were only spending 20 percent of their work hours on TB-related duties; the rest was spent dealing with general medicine and administrative tasks. Van Rie showed her results to the clinic staff. The next time she checked in with them, they had managed to make ends meet with the staff they already had.
Van Rie’s research regularly takes her to Sizwe Hospital in South Africa, which is devoted exclusively to treating MDR and XDR patients. (See “A Prison for the Sick.”) Public health officials all over the world are now facing the same problem: how to best treat people who are sick with drug-resistant strains.
In a January 2007 article in the Guardian, South African scientist Mary Edginton said, “You can look at it from two points of view. From the patient’s point of view, you are expected to stay in some awful place, you can’t work and you can’t see your family. You will probably die there. From the community’s point of view such a person is infectious. If they go to the shops or wander around their friends they can spread it, potentially to a large group of people.”
MDR-TB is transmitted in hospitals much more often than other types of TB, Van Rie says, partly because overcrowding and poor ventilation often turn hospitals into breeding grounds for the disease. So while Braunstein, Hickey, and other scientists search for a vaccine, Van Rie and others are looking for the solution to treating dangerously contagious (and often-terminally ill) patients with compassion and dignity.
“These patients feel whether you’re comfortable sitting with them or whether you’re scared of them,” Van Rie says. “And if you’re scared of them, you’d better do some other job. If you’re scared of them, you can’t do good research. Your heart has to be in it.”
Beth Mole was formerly a postdoctoral fellow in the medicinal chemistry and natural products division of the Eshelman School of Pharmacy.
Miriam Braunstein is an associate professor of microbiology and immunology in the School of Medicine. Her work is funded by the Burroughs Wellcome Fund, the National Institutes of Health (NIH), and Aeras. Tony Hickey is a professor of molecular pharmaceutics in the School of Pharmacy. His work is funded by the Bill and Melinda Gates Foundation. Annelies Van Rie is an associate professor of epidemiology in the School of Public Health. Her work is funded by NIH, the CDC, and the United States Agency for International Development. Myron Cohen, director of the Institute of Global Health and Infectious Diseases, provided background expertise for this story.