Responsible Conduct of Research (RCR)
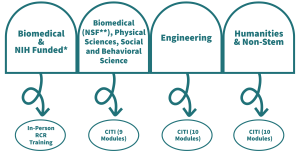
From September 1st, 2023, all UNC postdocs are required to undertake Responsible Conduct of Research (RCR) training in the first year of their appointment. There are two routes to acquire this training: 1) In-person or 2) Online (via CITI). Which format and modules you require will be determined by your funding source and field of research. Training is also mandated by all federal granting programs including NIH and NSF, and is strictly monitored. The Office of Postdoctoral Affairs offers an in-person course twice a year (Spring and Fall) that will satisfy the RCR training requirements for the NIH and NSF research grant applications.
* NIH funded postdocs will need to complete the in-person, 2-day RCR training.
** NSF-funded postdocs can take the online CITI modules outlined below to be NSF-compliant, but attending the in-person 2-day training will also satisfy NSF requirements and is encouraged.
In Person RCR Training Information
Fall Session: November
Spring Session: April
Next Session: Spring RCR Training: April 25th & 26th, 2024. Register Here.
Postdoctoral Scholars must complete all days to receive a certificate of completion.
This course is free and available for all UNC-CH Postdoctoral Scholars. Graduate students should inquire with the UNC Graduate School for appropriate RCR training options. NC TraCS also run an in-person RCR every summer (usually in July) which has a more clinical focus, but also meets the NIH RCR training requirements.
Online RCR Training Information
CITI: Collaborative Institutional Training Initiative is an online portal for UNC RCR training. The CITI homepage can be accessed at https://about.citiprogram.org/en/homepage/. The blue login button will take you to a page where you can access RCR training via “Single Sign On” (SSO). Select “LOG IN THROUGH MY ORGANIZATION.” You can scroll down until you find University of North Carolina at Chapel Hill or simply search using Chapel Hill. Use your ONYEN to log in.
Once logged in you can view courses available to you. You will want to select an RCR course that best suits the area or research or research training you are engaged. If you are unsure consult your research mentor, department or program.
Your choices are Biomedical Responsible Conduct of Research; Physical Science Responsible Conduct of Research; Responsible Conduct of Research for Engineers; Social and Behavioral Responsible Conduct of Research; or Responsible Conduct of Research for Humanities/Non-Stem. Each RCR course has between 9 and 10 modules that require successful completion.
Most modules will take about 10-20 minutes to complete. Continuing education is required every 3 years. UNC-CH is automatically notified once you have completed the CITI training and your name will appear in the UNC Research Training database within 48 business hours. It is not necessary to forward your completed CITI course information to the University. To generate a training completion certificate, go to the UNC Research Training Database. For additional information, please visit here.
You will need to successfully complete all of the associated modules selected to meet both UNC and NSF requirements. Please note that the CITI RCR courses will not satisfy RCR training requirements for the NIH.
Objectives and Goals
The primary objective for the UNC Postdoctoral Scholar Three Day Course is to address RCR topics such as Publication Practices and Responsible Authorship, Mentor/Trainee Responsibilities, Research Misconduct, and Communication and Difficult Conversations.
The program’s goals are to:
- Provide a basic training in research ethics which will focus on ethical decision making and encourage postdoctoral scholars to think about the impact of their research and decisions on society.
- Introduce interdisciplinary views into the ethics training
- Create a community that promotes the importance of ethical issues and fosters open discussions among postdoctoral scholars as well as faculty, students and staff.
The following topics are covered during virtual/in-person instruction, totaling at least 8 hours or more of contact:
- Conflict of interest
- Policies regarding human subjects, live vertebrate animal subjects in research, and safe laboratory practices
- Mentor/mentee responsibilities and relationships
- Collaborative research
- Data management, sharing and ownership
- Research misconduct and policies for handling misconduct responsible authorship and publication
- Researcher as a responsible member of society, contemporary ethical issues
If you are interested in learning more, contact OPA at opa@unc.edu.
Additional Information and Resources
Relevant Links and Articles:
Office of Research Integrity newsletters
For more information about university policies, please visit Research Compliance Program website for Responsible Conduct of Research.
NAE workshop -Ethics Educations and Scientific and Engineering Research: What’s Been Learned? What Should Be Done?
UNC Resources:
Report Ethics Concerns to Ethics Point
Quick links
- To Report a Concern Anonymously: Ethics Point
- For HIPAA comments or questions: hipaa@unc.edu
Other Resources:
The National Academy Press Being a Scientist, A Guide to Responsible Conduct in Research: Third Edition (2009) available online as a free .pdf
NPA Postdocket (Winter 2011) — Train-the-Trainer: A Different Approach to Responsible Conduct of Research, by Stephen Fuchs and Sibby Anderson-Thompkins
The Office of Research Integrity Guide to Ethical Writing
List of Participants that Completed Advanced Training in Responsible Conduct of Research:
Responsible Conduct of Research Training – Fall 2023
Responsible Conduct of Research Training – Spring 2023
Responsible Conduct of Research Training – Fall 2022
Responsible Conduct of Research Training – Spring 2022
Responsible Conduct of Research Training – Fall 2021
Responsible Conduct of Research Training – Spring 2021
Responsible Conduct of Research Training — Fall 2020
Responsible Conduct of Research Training — Spring 2020
Responsible Conduct of Research Training — Spring 2019
Responsible Conduct of Research Training — Spring 2018
Responsible Conduct of Research Training — Fall 2017
Responsible Conduct of Research Training — Spring 2017
Responsible Conduct of Research Training — Fall 2016
Responsible Conduct of Research Training — Spring 2016
Responsible Conduct of Research Training — Spring 2015
Responsible Conduct of Research Training — Fall 2014
Responsible Conduct of Research Training — Spring 2014
Responsible Conduct of Research Training — Fall 2013
Responsible Conduct of Research Training — Spring 2013
Responsible Conduct of Research Training — Fall 2012
Responsible Conduct of Research Training — Spring 2012
Responsible Conduct of Research Training — Fall 2011