By investigating the behavior of stem cells in a microscopic worm, Kacy Gordon’s lab hopes to produce useful knowledge at larger scales of biological complexity.
Basic biology is anything but basic. And Kacy Gordon just got a nearly $2 million National Institutes of General Medical Sciences grant to prove it. But before we dive into the biology professor’s research, we should get to know her research subject — wait, now it’s subjects. That’s almost how fast the barely-visible-to-the-unaided-eye Caenorhabditis elegans worm multiplies.
“If you put one on your Petri dish on Monday, by the end of the week you will have 300,” Gordon says.
The hermaphroditic C. elegans worm is ideal for efficiently studying a life cycle, as it matures and reproduces in one to five days. It is also a model organism — an animal that shares main cellular components with other animals like mice and even humans. Its see-through skin allows researchers to see how the worm’s cells function in their natural arrangements by looking from the outside in.
C. elegans is a longstanding example of how stem cells grow, develop, and function. In fact, what’s known about the way human stem cells communicate and move was first discovered in worms and later found to be important in other tissues as well. More generally, basic research in cell biology has improved our understanding of diseases and how to treat them. There may be a long path from basic research to clinical intervention, but without the former, the latter wouldn’t exist.

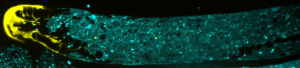
Gordon’s lab studies the germline, where stem cells multiply and form sperm and eggs that contain genetic information passed on from one generation to the next. They want to learn how other cells interact with stem cells to cause either proliferation or transformation into a reproductive cell, ultimately uncovering which chemical signals shape a cell’s fate. Seeing these interactions requires the use of some visual aids.
“We modify the worm stem cells with a genetic editing technique called CRISPR so they express a fluorescent protein that was developed from a jellyfish,” Gordon explains. “With this glow, we can track cell interactions and movements in real-time through a microscope.”
This genetic modification slightly impacts the cells’ ability to function normally, according to Gordon. She equates it to a person holding a brightly colored helium balloon in a large crowd. They could be easily identified without their movement being significantly impacted by the balloon’s presence. So, generally, the proteins can do their jobs while Gordon and her team watch.
Tracking the stem cells’ movements is important because part of becoming a sperm or egg cell involves leaving a very hospitable microenvironment for stem cell growth called a niche. While it may be cozy in the niche, it isn’t ideal for stem cells to stay there and divide indefinitely. That can produce tumors and reduce fertility. So, how do stem cells know when it’s time to leave the niche?
“We used to think that maybe cells were randomly signaled and pushed out of the niche to go on and form sperm and eggs,” Gordon says. “But we’re discovering there’s more order to it, and we think sheath cells play a bigger role than previously thought.”
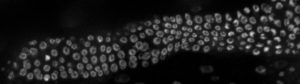
Sheath cells are long and skinny — so skinny that other researchers had overlooked them in the past. They are known to be important in stem cell proliferation and creating an appropriately sized gonad — the organ that contains sperm or eggs. But the “how” and “why” were unknown, and their shape makes them difficult to study. Gordon’s lab is now using CRISPR to tag specific proteins of the sheath cells to track their movements in conjunction with stem cells, revealing exciting findings.
“We can now see that sheath cells grab stem cell daughters and migrate them from the niche,” Gordon says. “We’ve also seen that sheath cells grow exponentially during development and take on the size and shape of the germline, indicating there is signaling between the two components.”
These findings helped Gordon secure nearly $2 million in funding over five years to support her lab’s work. The R35 grant is open-ended funding, meaning the researchers aren’t required to lay out specific theories and goals, but more of a set of questions they want to find answers to. The flexibility is necessary when mapping out biological processes as they are being discovered, allowing the lab to follow data instead of a strict set of plans.
While Gordon’s team has made great advancements in our understanding of germline stem cell growth and movement, there’s still much more to explore. And that takes time.
“The value of this type of basic research is not apparent at the time it is being done, and sometimes not for years later,” Gordon says. “Really important discoveries come from studying how cells work and that’s what keeps us moving forward and hopeful.”
Kacy Gordon is an assistant professor in the Department of Biology within the UNC College of Arts and Sciences.
